Here is a tale of how an inherited health issue becomes clearer the deeper one looks into a family tree. (A lesson from a family tree I have worked on.)
Introduction
It starts with looking at the recent adult generation of a family tree I was working on. I knew from information from the family that 3 amab first cousins of that generation were all diagnosed with cancer and died before age 50:
- 1 case of acute leukemia in late adolescence
- 1 case of non-Hodgkins lymphoma before age 25
- 1 case of colorectal cancer at age 40
There was also possibly an afab first cousin with cancer, but I couldn’t get specifics, and that person might not have been a step-cousin.
That… seems like rather a lot of people with cancer. So I took a look at the next generation back.
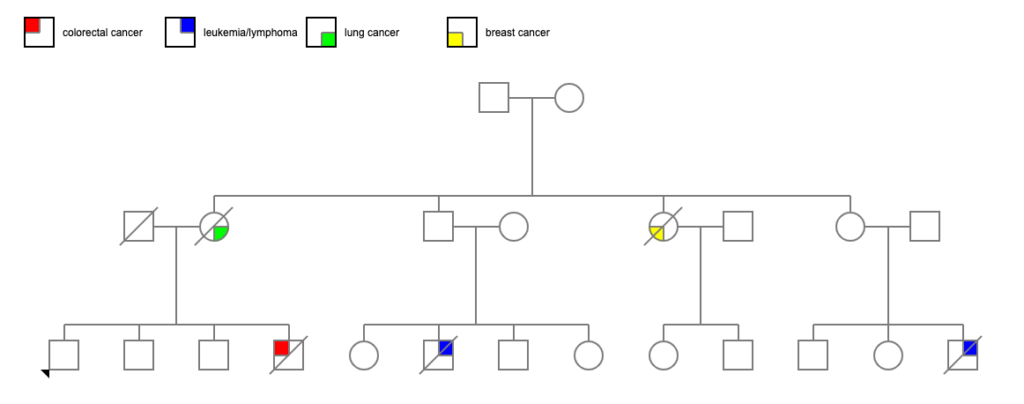
Two of the 4 siblings died of cancer — one of breast cancer diagnosed before age 60, and one of lung cancer around age 80. In general, 1 in 3 to 1 in 4 USians will develop cancer at some point in their lives, so 2 of 4 is a bit above average. Certainly noticeable considering the generation after them.
So I looked at the parents of those 4 siblings.
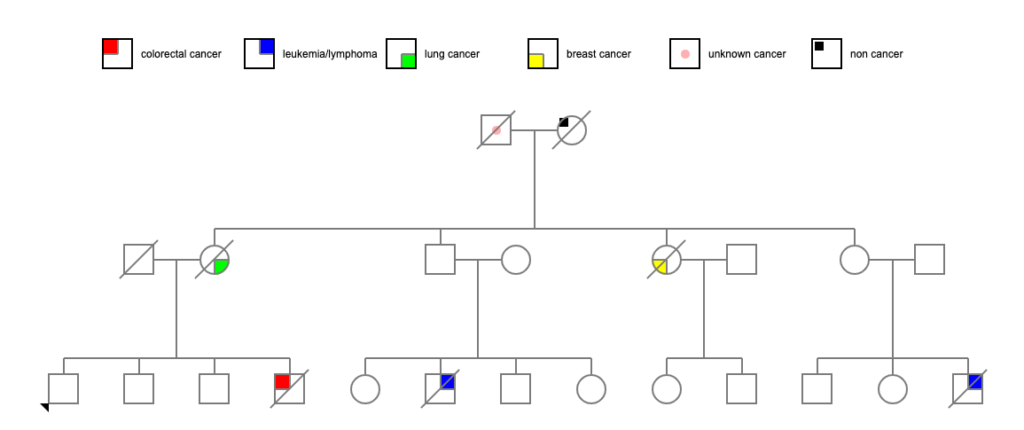
Their mother died at 81 of heart failure. However, their father (let’s call him Pete) died at age 48 due to generalized metastatic cancer — no information on the primary cancer. (He apparently died during a minor surgical procedure, and when they did the autopsy, they found that he had generalized metastasis. The death certificate indicated that his cervical lymph nodes were particularly affected. Lymphoma? Or just metastasis from some other primary cancer?) If we presume that there is a genetic cause of all these cancers, it seems obvious from which parent it might have come!
I was able to uncover death certificates for Pete’s parents. Both of them died fairly young, which was an intriguing start. But one of the problems with death certificates, as I think I’ve mentioned before, is that some doctors are incredibly literal. “John Doe died of pulmonary edema” — what caused John’s pulmonary edema? Was it heart failure? Was it cancer? Was it an infectious disease? Who knows?
Pete’s father Michael died at age 58 of a pulmonary embolism and endocarditis, and had a history of apoplexy (stroke) a year earlier. That seems straightforwardly cardiovascular enough (though cancer can increase the risk of stroke by altering the clotting of the blood). Pete’s mother Mary died at age 42 of a cerebral embolism in the middle of the 1918 flu pandemic (October 14, 1918) and was living near Philadelphia, which was one of the worst-hit cities in the US. Influenza, like many other viruses, can increase the risk of stroke, and a lot of doctors weren’t adding influenza as a cause of death on certificates at the time. So we may have an answer there.
A quick check one generation back provided some more data:
Michael’s father died at 75 of pulmonary edema with a contributing factor of hypertrophy of the prostate. Overgrowth of the prostate in a 75-year-old man in 1923 could have been benign hypertrophy OR it could have been prostate cancer. There’s really no telling, unfortunately.
Michael’s mother died at 64 of “ruptured compensation,” which is either heart failure or decompensated valvular disease of the heart. That’s fairly clearly cardiac. Sadly, his parents were immigrants from a country with no easily-accessible online data, so I can’t hunt more medical history there.
Mary’s father died at 85 of arteriosclerosis. Her mother died at 49 of uremia, which wasn’t all that unusual in 1899 — it was a common cause of death for those with diabetes, for instance, or any of several other conditions that might injure the kidneys. And they too were immigrants, where information stops.
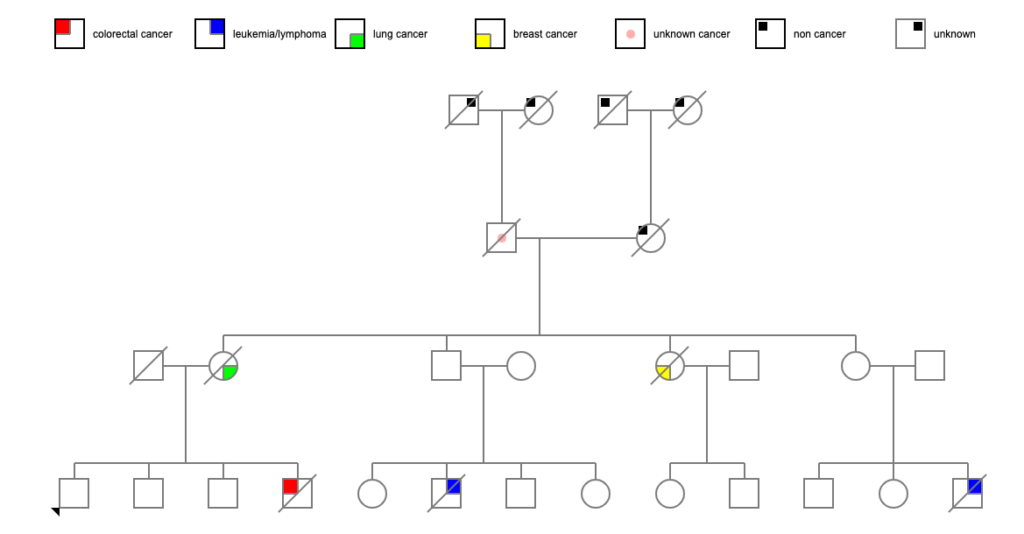
Let’s Check the Siblings
Back down the tree to Pete. He had a bunch of siblings! Some more data:
- Jim, who died at 61 of metastatic colon cancer
- Catherine, who died at 2 months of pneumonia
- Mary, who died at 70 of a sudden heart attack
- Frank, who died at 4 of “hemorrhages”
- Claire, who died at 6 months of lobar pneumonia
- Jean, who died at age 86 of unknown causes
- Michael, who died at age 1 years of meningitis and pneumonia
- Joe, who died at age 84 of unknown causes
Let’s take a quick run down Jim’s tree, since he’s the most obvious person who died of cancer in adulthood. He had 3 children:
- Jean, who died at 74 of unknown causes, though her obituary suggested donations to the “bone marrow unit” of the local hospital, which suggests blood cancer; Jean had 1 child::
- Sandra, who died at age 59 of leukemia, and had 2 children:
- Brian, who died at age 21 of leukemia
- Nan, who is still alive
- Sandra, who died at age 59 of leukemia, and had 2 children:
- Bob, who died at 83 of melanoma; Bob had 3 children:
- Bob Jr, who died at age 41 of anaphylactic shock from multiple yellowjacket stings
- Cathy, who is still alive
- Cheryl, who is still alive
- Darcy, who is still alive
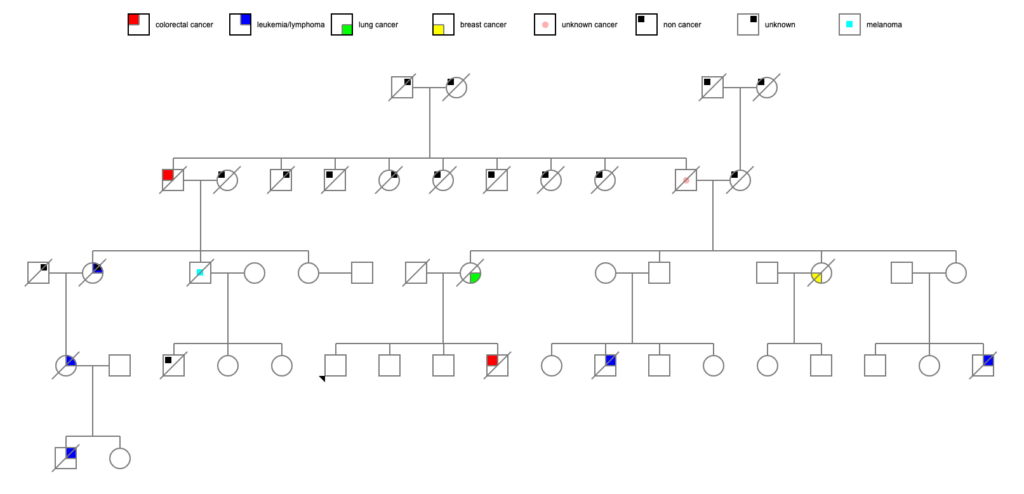
Wow, okay, Jean, her daughter, and her grandson certainly seem to factor into the equation here. Bob, at 83 with melanoma, might or might not be a related data point.
That’s a lot of cancer in 2 family branches, happening to relatively young people, especially when one compares to the unaffected family branches, where most people died of cardiovascular disease. Striking, you might call it if you were a genealogist and a geneticist.
Many cancer-predisposing genes are inherited in an autosomal dominant fashion, meaning that only one copy needs to be inherited to manifest. But these genes are also influenced by environmental factors, which is why they generally only increase the risk of developing cancer, rather than making it an absolute future. They also tend to mutate fairly often: for instance, when inherited BRCA1 mutations are found in someone with breast or ovarian cancer, about 40% of the time that person doesn’t have a family history of cancer. There was a decent chance, when all I knew about was Pete and his descendents, that Pete was gene patient zero..
However, given what I found with Pete’s brother Jim and his descendents, we’re probably looking at a gene that was inherited from one of their parents, both of whom died youngish of apparently noncancerous causes. Is there any way to look into that? Siblings again!
Pete’s mother Mary had a couple of siblings, but the only one I could find a death date on was a woman who died of a stroke just a couple years after Mary. Strokes were really going around in that family! The 1918-1920 period was pretty stressful.
Pete’s father Michael had more traceable siblings:
- Mary, who died at 74, and of her 7 children, 1 died in infancy, 1 died at age 38 (“after a brief illness”), 1 died at 53 (“following a short illness”), and the rest died between the ages of 76 and 100.
- Dan, who died at 77, and of his 3 children, 1 died at 32 after “an illness of 10 weeks,” 1 died at 73, and the last died at 97.
- Tony, who died at 68 of emphysema and bronchitis, and had no children.
- Lou, who died at 80 after a “brief illness,” and of his 5 children, 1 died at 76 after a “lengthy illness,” 1 died at age 2 of enterocolitis, 1 died at age 68 of unknown causes, 1 died at 69 of a heart attack, and 1 died at 83 of Alzheimer’s.
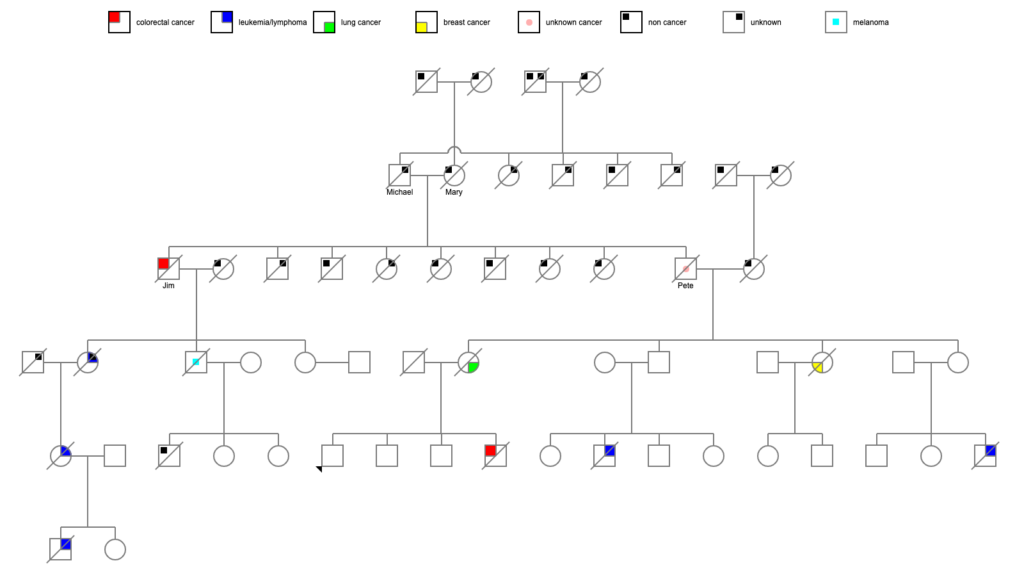
There is nothing jumping out as a potential run of cancer in this part of the family, with the possible exceptions of the 2 mysterious 30-somethings and 1 50-something dying after brief/short/10-week illnesses. Brief illnesses in 50-somethings are usually cardiac, but in 30-somethings, it’s a big question mark. But none of the 5 siblings appear to have died young or markedly of cancer. If we’re postulating an autosomal dominant gene, then about half of the siblings should have turned up with something. Granted, we don’t know about actual cause of death for 3 of them.
I must reluctantly come to the conclusion that we don’t have sufficient data to conclude which parent gave Jim and Pete their hypothetical cancer gene.
More Hypotheses
All right, if we’re going to go down the track of hypothetical cancer genes, what are we looking at?
Our only certain data points are:
- 1 case of generalized metastatic cancer of unknown origin (amab)
- 1 case of melanoma (amab)
- 1 case of breast cancer (afab)
- 1 case of lung cancer (afab)
- 2 cases of colorectal cancer (amab)
- 4 or 5 cases of leukemia or lymphoma (3 amab, 1 or 2 afab)
The melanoma and lung cancer can almost be written off as incidentals: the fellow who died of melanoma happened to have an address in a known beach town, the woman who died of lung cancer was a nearly lifelong smoker, both of them over 80. Which leaves us leukemia/lymphoma predominant, with colorectal and breast cancer in the mix. Not a lot of data to work with. The sex balance is interesting: 6 males, 3 females. Should we be looking at an X-chromosome-linked gene? Or is there some protective quality in action? Or do we just have a random assortment that happens to have an imbalance? Again, not a lot of data. If I could get information on the many unknowns, it might give us more insight.
This is, at this point, not the pattern of either of the BRCA gene mutations. In both BRCA1 and BRCA2, there is an overwhelming majority of breast and ovarian cancers (along with closely related cancers like fallopian tube cancer and primary peritoneal cancer), with an increased chance of pancreatic cancer and prostate cancer. Another cancer syndrome that comes up at the top of literature searches is Li-Fraumeni syndrome (a mutation in the gene TP53), but it has cancer types not seen in this family: sarcoma, brain cancer, and adrenal cancer. It’s also almost completely penetrant in its classic form, meaning that people who inherit the mutation are going to get cancer if they live long enough.
This is likely a gene mutation that is incompletely penetrant — just in the initial family branch, there are apparent generation skips — but most likely autosomal dominant inheritance, given the way it can show up in every generation. (The family would have to have remarkably crap luck to keep marrying mutation carriers from other families.) There are a lot of familial cancer syndromes out there, and more being found all the time. Just running cancer predisposition searches on Online Mendelian Inheritance in Man (OMIM) can be overwhelming: a search for “leukemia predisposition” brings back 1900 results.
I’m not a cancer geneticist by a long shot, but I can see the outlines of patterns in family history research. This is just one family that I’ve spotted with such an issue — there are others that I’ve run across in other families that show even more definite patterns, ones where I can say, “Oh, I bet there’s a BRCA issue here,” or, “Looks like one of the familial colorectal cancer genes.”
This is just varied enough to be hard to make an untutored call. It looks like a pattern. It feels like a pattern. But I suspect that a cancer geneticist would probably want to do some gene sequencing on the family to spot the source of the issue, and to be able to test the family members who are at risk. And get a paper from it, of course.
